TransportGut
Multi-scale modelling of transport in the gastrointestinal tract
funded by Agence Nationale de la Recherche - ANR-21-CE45-0015
The Project
The gastrointestinal tract involves many biological, chemical and physical phenomena to secure the absorption of nutrients from our food. Also, specific sites of the digestive mucosa are gateways to our immunologic system which pave the way for the development of innovative oral therapeutic strategies. These strategies are based on the encapsulation of drugs in nano- or micro- particles or the administration of bacteria, which would target these sites in order to induce an immune response. However, a major scientific barrier is to be able to predict the flow of these "micro-particles" and thus control the dose absorbed by our body.


The objective of TransportGut is to develop a predictive and comprehensive modelling of the transport of microparticles in the gastrointestinal system.

The challenge of such a model is to account for the different specificities of the physical environment of the digestive tract on the phenomena of transport and mixing. On the one hand, transport and mixing are controlled by the mechanical activity of the smooth muscles of the intestinal mucosa, on both macroscopic and microscopic scales. On the other hand, this activity varies according to the time scales considered. Several scales are thus relevant: the microstructures of the mucosa, the isolated organ and along the digestive system. Mixing at large scales are probably controlled by mixing at small scales. There is currently no particle transport model that takes into account these different scales.
Based on experiments at the interface of physiology and fluid mechanics and numerical simulations of flows, we propose to develop an analytical model of transport connecting these different scales. We will develop experiments on animal models to study the transport of particles along the digestive system and in the vicinity of microstructures of the intestinal mucosa. These experiments will be used to simulate numerically the coupling between flows at microscopic and macroscopic scales in order to understand the role of active and microstructured interfaces on the transport and mixing of microparticles. All of these data from experiments and numerical simulations will make it possible to build analytical and simplified models of the transport and mixture of particles at different spatial and temporal scales. This model would predict the spatiotemporal dispersion of particles in order to be a decision-making tool for the pharmaceutical industry, but also to understand the fundamental mechanisms that govern the spatial structure of the intestinal microbiota.
The reserachers of the TransportGut team are based in three different labs in Paris and Grenoble:
Laboratoire Rhéologie et Procédés (LRP): Experiments in complex flow at macroscopic and microscopic scales, numerical modeling of flows in the gastrointestinal tract.
Laboratoire Jean Perrin (LJP): Theoretical modeling of transport phenomena of microparticles in the small instestine.
TIMC: Physiology of smooth muscles and small intesinal motility.
 From left to right: Stéphane Tanguy , Faisal Ahmad, Dacil Yanez Martins, Rohan Vernekar,
Clément de Loubens, Claude Loverdo, Martin Garic
From left to right: Stéphane Tanguy , Faisal Ahmad, Dacil Yanez Martins, Rohan Vernekar,
Clément de Loubens, Claude Loverdo, Martin Garic
Claude Loverdo, CRCN CNRS, LJP. Claude is developing simplified models of particle transport and mixing in the gut connecting different spatail and temporal scales, based on experiments that combine physiology and fluid mechanics, as well as numerical simulations of flows.
Clément de Loubens, CRCN CNRS, LRP. Clément's research focuses on understanding complex flow phenomena in the gut through the use of CFD models, ex-vivo experiments, and in-vitro experiments.
Stéphane Tanguy, MCF UGA, TIMC. Stéphane is developing specific experiments to better understand the physiological function of the small intestine smooth muscle at different scales - the GI tract, individual organs, and villi.
Martin Garic, PhD Sorbonne Univ., LJP. Martin's PhD work involves developing analytical models for the transport and mixing of particles in the GI tract. He bases these developments on CFD models and physiological experiments.
Faisal Ahmad, PhD UGA, LRP, TIMC. Faisal's PhD work focuses on developing computational models for complex fluids at both the organ and villi scales. In addition, he is also developing an experimental bench to study small intestine motility at both the macroscopic and microscopic scales.
Rohan Vernekar, Post-Doc CNRS, LRP. Rohan is building the intestinal flow solver, using the lattice Boltzmann method. He is also carrying out simulations to study transport at the sub-organ as well as the villi scales, and is co-supervising PhD students in the group.
Dacil Yanez Martins, PhD CNRS, LRP, TIMC. Dacil's work involves developing experiments that couple gut physiology and microfluidics to understand the role of gut motility, at different scales, in the mixing and transport of microparticles.
Hydrodynamics induced by villi-wave motions.
We have explored how wave-like, (pendular) contractions in the small intestine, specifically in the rat duodenum, influence fluid movement and mixing. Our simulations reveal that these motions create two distinct flow layers: a mixing boundary layer near the walls with swirling motion and an advecting layer above with steady, unidirectional flow. Even in highly viscous conditions, the steady axial flow persists, driven by coordinated villi motion breaking time-symmetry in Stokes flow conditions. We have identified key parameters that govern the boundary layer height as well as determine whether mixing or propulsion dominates, with an optimal range for maximizing axial pumping. Finally, our findings suggest that this bio-inspired motion could be applied in microfluidic systems for efficient mixing and flow control.
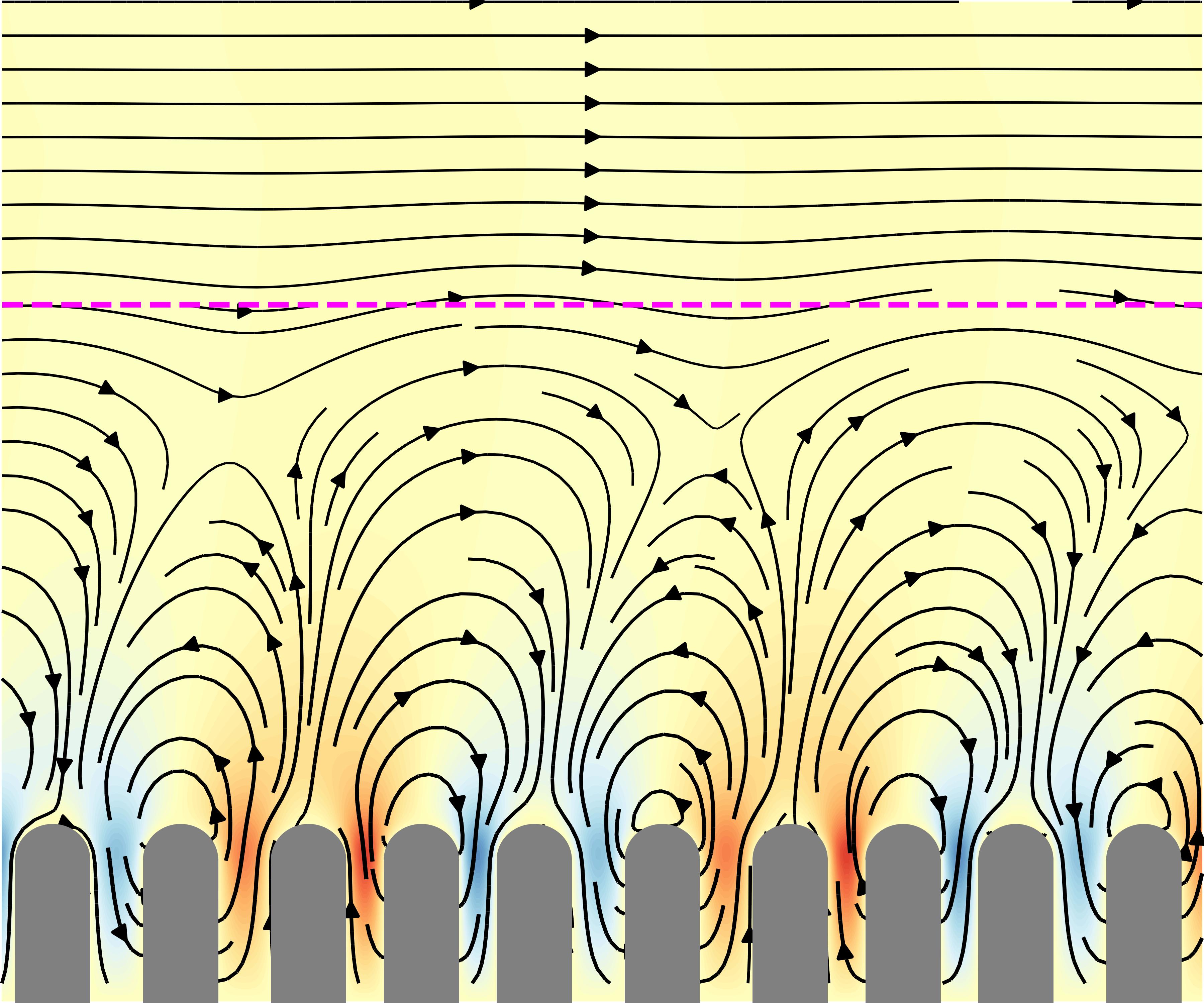 Flow field generated by the pendular wave of villi, showing both a mixing layer near the villi tips and an advection layer in the middle of the lumen.
Flow field generated by the pendular wave of villi, showing both a mixing layer near the villi tips and an advection layer in the middle of the lumen.
Organ bath for assessing the mechanosensitivity of the small intestine.
Our organ bath system is designed to quantify ex vivo small intestine motility under controlled mechanical and fluidic conditions. It allows the application of longitudinal stress or strain using an air-bearing system while precisely controlling infusion through regulated pressure differences or flow rates. High-resolution imaging enables detailed tracking of intestinal length variations and the generation of spatio-temporal motility maps. This setup provides valuable insights into the mechanosensitivity of the gut, helping to distinguish between different motility patterns and their response to mechanical stimuli.
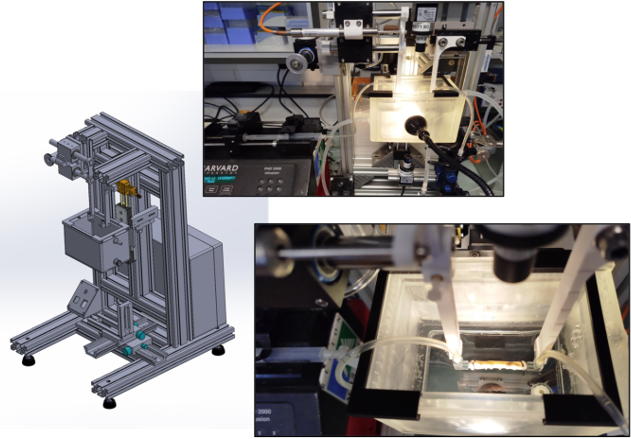 Organ bath to study small instine motility at macro- and micro- scales. Designed by M. Karrouch, D. Blésès, F. Hugenell (LRP).
Organ bath to study small instine motility at macro- and micro- scales. Designed by M. Karrouch, D. Blésès, F. Hugenell (LRP).
A lattice-Boltzmann model for the flow of yield stress fluids at an organ scale applied to human defecation.
A lattice-Boltzmann model was developed to simulate the flow of yield stress fluids at the organ scale, based on in vivo motility data. The model was applied to study rectal evacuation in patients with normal and impaired defecation, quantifying velocity, pressure, and stress fields during the defecation of a neostool with rheology similar to that of soft stool. This allowed to identify biomechanical and rheological facors affecting normal and pathological defecatory function in these patients.
 Left: Simulated pressure field during defecation of a neostool with rheology similar to that of soft stool.
Right: X-ray defecography of the corresponding patient.
Left: Simulated pressure field during defecation of a neostool with rheology similar to that of soft stool.
Right: X-ray defecography of the corresponding patient.
Secondary flow generated by the oscillation of small intestine villi.
We modelled flow and mixing induced by a simplified version of villi motility. The model shows the emergence of a secondary flow phenomenon, a steady streaming flow, in a micro-mixing layer adjacent to the villi. The intensity of this flow could control the transport of microparticles, such as bacteria, in an area close to the villi, on long time scales.
 The steady Lagrangian streamlines that result from the oscillation of an infinite array of small intestine villi.
The vortices observed in the micro-mixing layer near the tip of the villi are also depicted.
The steady Lagrangian streamlines that result from the oscillation of an infinite array of small intestine villi.
The vortices observed in the micro-mixing layer near the tip of the villi are also depicted.
Hydrodynamics in a villi-patterned channel due to pendular-wave activity
Abstract
Inspired by the motility of small intestine, we investigate the flow generated by a propagating pendular-wave along the walls of a symmetric 2D channel lined with elongated villi-like microstructures. The rigid villi follow simple harmonic axial motion, but with a phase lag to their neighbours, resulting in travelling intervillous contractions. We use lattice Boltzmann simulations to resolve the flow around the villi and the luminal channel space, sampling small to moderate Womersley numbers. We analyse an emergent `mixing' boundary layer in the lumen, separating two distinct flow regions. A mixing region is formed above the villi, characterised by semi-vortical flow patterns, which travel along with the wave. In the channel centre, unidirectional axial flow emerges, flowing opposite to the imposed wave direction, even in the Stokes flow regime. This behaviour contrasts with canonical wave driven peristaltic flow (Jaffrin & Shapiro 1971). The fluid trapped in the intervillous gaps is forced to take a non-reciprocal path due to the travelling pendular-wave, which breaks time-symmetry in the Stokes regime. We propose an effective velocity boundary condition, incorporating perturbations due to the presence of the villi. In the inertial regime, the boundary layer shrinks due to dynamic flow confinement arising from increased oscillatory flow inertia. We identify the Stokes to inertial transition, and find phenomenological scaling laws for the boundary layer decay and axial and radial fluid fluxes. Our results not only advance the understanding of transport within the gut but also suggest a novel way for microfluidic flow control in confined channels.Imaging small intestinal motility at macro and micro scales
Abstract
Our main objective is to develop an image technique that permit the quantification of the smallintestine motility at the scale of the organ and at the scale of the villi in order to understand the interplay between smooth muscle motility and villi movement.Investigating human defecation by coupling fluid mechanical modeling and X-ray video-defecography
Abstract
To gain new insights on the biomechanics of human defaecation, we developed patient-specific simulations of rectal evacuation of yield stress fluids based on X-ray video-defecographies with a neostool. First, we segmented automatically X-ray videos with a Convolutional Neural Network. Then, flow, pressure and stress fields were simulated by lattice-Boltzmannmethods for yield stress fluids. Lastly, we applied the model to simulate normal defecation, and impairedd efecations in absence or in presence of obstructive phenomena (rectocele).Flow simulations of rectal evacuation: towards a quantitative evaluation from video defaecography
Abstract
Mechanistic understanding of anorectal (patho)physiology is missing to improve the medical care of patients suffering from defaecation disorders. Our objective is to show that complex fluid dynamics modelling of video defaecography may open new perspectives in the diagnosis of defaecation disorders. Based on standard X-ray video defaecographies, we developed a bi-dimensional patient-specific simulation of the expulsion of soft materials, the faeces, by the rectum. The model quantified velocity, pressure and stress fields during the defaecation of a neostool with soft stool-like rheology for patients showing normal and pathological defaecatory function. In normal defaecation, the proximal–distal pressure gradient resulted from both the anorectal junction which formed a converging channel and the anal canal. The flow of the neostool through these anatomical parts was dominated by its shear-thinning viscous properties, rather than its yield stress. Consequently, the evacuation flow rate was significantly affected by variations in pressure applied by the rectum, and much less by the geometry of the anorectal junction. Lastly, we simulated impaired defaecations in the absence of obvious obstructive phenomena. Comparison with normal defaecation allowed us to discuss critical elements which should lead to effective medical management.Towards an assessment of rectal function by coupling X-ray defecography and fluid mechanical modelling
Abstract
Despite the numerous available clinical investi-gation tests, the associated alteration of quality of life and the socio-economic cost, it remains difficult for physicians to identify the pathophysiological origins of defecation disorders and therefore to provide the appropriate clinical care. Based on standardized dynamic X-ray defecography, we developed a 2D patient-specific computational fluid dynamic model of rectal evacuation. X-ray defecography was carried out in a sitting position with a standardized paste whose yield stress matched that of soft human feces. The flow was simulated with lattice-Boltzmann methods for yield stress fluids and moving boundary conditions. The model was applied for a patient with a normal recto-anal function. We deduced from the flow field that the main flow resistance during the defecation was due to the extrusion of the paste through the anal canal. We calculated also from pressure and stress fields the spatio-temporal evolution of the wall normal stress. This latter highlighted a gradient from the proximal to the distal part of the rectum. We discussed how this new set of hydrodynamical and biome-chanical parameters could be interpreted to gain new insights on the physiology of defecation and to diagnose underlying evacuation disorders. Clinical relevance - If confirmed, our approach should allow clinicians to obtain other parameters from a classic clinical examination and thus better adapt the response of clinicians to the defecation disorders observed in patients.Steady streaming flow induced by active biological microstructures; application to small intestine villi

Abstract
Physiological transport of fluid at small scales is often achieved by microscopic active fingerlike structures. It is recognized that they have to move in a non-symmetric fashion in order to break the symmetry of creeping flow and to induce a net movement of the fluid. However, in the limit of low, but non-vanishing, Reynolds number, irreversible flow on long time scales could also be generated by symmetric oscillations of these microstructures. Inspired by small intestine villi, we reported three dimensional direct numerical simulations of the irreversible part of the flow, namely steady streaming flow (SSF), generated by an array of oscillating fingerlike structures. In order to capture these second order flow phenomena, the algorithm was based on a combination of lattice-Boltzmann methods with two relaxation times and the smoothed profile method. SSF was confined inside a steady viscous boundary above the villi. Two steady vortices at the tip of the villi characterized this flow which induced mass transfers between the bulk and the periphery. Strikingly, the spatial extension of these vortices was not solely governed by the Stokes boundary layer but also by the lateral confinement between the villi. Moreover, secondary vortices outside the steady boundary layer were also observed. These findings were rationalized in a state diagram showing three regimes of SSF. Finally, orders of magnitude showed that SSF should contribute to the transport of particles, such as bacteria or nano-particles, on a layer a few hundred micrometers above the villi and on a time scale of few minutes.Supplementary material from "Flow simulations of rectal evacuation: towards a quantitative evaluation from video defecography"
